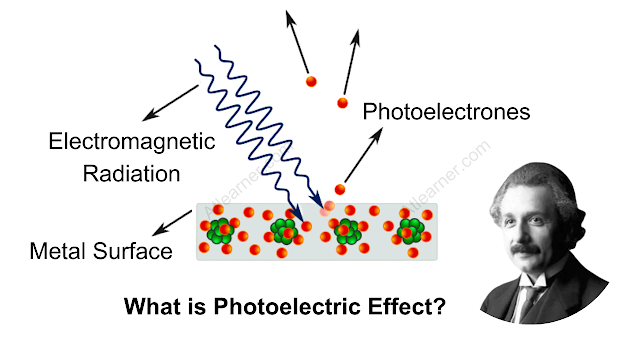
What is Photoelectric Effect?
The photoelectric effect is a phenomenon in which electrons are emitted from a metal surface when it is exposed to light. These emitted electrons are called photoelectrons. However, it is very important to note that the emission of photoelectrons and the kinetic energy of the emitted photoelectrons are dependent on the frequency of light that is incident on the metal surface.
The process by which photoelectrons are emitted from a metal surface due to the action of light is commonly called photoelectric emission.
We will discuss more about photoelectrons, and photoelectric emission further, but to truly know the significance of the photoelectric effect it is essential to first delve deeper into its historical context.
Discovery of the Photoelectric Effect
Albert Einstein's profound insight and groundbreaking theory revolutionized our comprehension of light and its interaction with matter. But before he explained the photoelectric effect, several scientists made similar observations but were unable to clarify the concept.
For example, Max Planck's work on black-body radiation and his concept of energy quanta set the stage for the Photoelectric Effect's interpretation. Hertz's experiments with electromagnetic waves paved the way for further investigations into the nature of light.
In 1887, the photoelectric effect was first noticed by German physicist Heinrich Rudolf Hertz, while conducting experiments related to radio waves. He noticed that in a vacuum tube, sparking takes place when two metal electrodes are shone with ultraviolet light, and there is a voltage change because of the light. He thought that the UV rays generated a large number of charged particles in the tube, which can be identified as the cause of sparking.
In 1902, Philip Lennard provided additional clarification regarding the relationship between electricity and light, further proving the theory of the photoelectric effect. Lenard's experiments and controversial views sparked a debate that ultimately led to a deeper understanding of the Photoelectric Effect.
Now before going to Einstein's photoelectric equation let's know some basic concepts about the photoelectric effect.
Important Concepts in Photoelectric Effect
Photoelectric emission:
When light of a suitable wavelength is incident on a metal surface, electrons are ejected from it, this phenomenon is called photoelectric emission.
Electrons emitted in photoelectric emission are called photoelectrons. With suitable arrangements, a unidirectional current of photoelectrons can be produced, the resulting electric current is called photoelectric current.
Work Function:
The minimum amount of energy needed for an electron to escape from the surface of a metal is called the work function of the metal. It is represented by the symbol W0.
This work function parameter depends only on the nature of the metal, not on how the electron gained energy. It is usually measured in units of electron volts (eV).
Alkali metals such as sodium, potassium, etc. have lower work function than other metals, but nowadays some alloys are used which are more convenient for photoelectric emission.
Stopping Potential:
The minimum negative potential or voltage applied to the anode to stop the photoelectric current is called the cut-off or stopping potential. It is represented by the symbol V0.
The stopping potential (V0) does not depend at all on the intensity of the incident light. As the incident light intensity increases only the value of the photoelectric current increases.
Irrespective of the intensity of the incident light, the value of the stopping potential remains the same for the light of a particular frequency.
Threshold frequency:
The minimum frequency of light that emits electrons or causes photoelectric emission from a metal surface when light falls on it is called threshold frequency. It is represented by the symbol 𝜈₀.
The maximum wavelength corresponding to this minimum frequency (𝜈₀) is called the threshold wavelength (𝜆₀ = c/𝜈₀). (where c = speed of light).
The higher the frequency of the incident light, the higher the stopping potential or maximum kinetic energy of the photoelectrons. This is why practically UV light is always applied to metal surfaces because UV light has a higher frequency and shorter wavelength than visible light.
Alkali metals (sodium, potassium, cesium, etc.) emit photoelectrons even when exposed to light of very low frequency.
Explanation of Photoelectric Effect
Max Planck proposed that the energy of electromagnetic radiation (like light) is quantized, meaning it comes in discrete packets called "quanta".
He introduced the Planck constant (h) to describe this relationship: E = h𝜈, where E is the energy, h is Planck's constant, and 𝜈 is the frequency of the radiation.
Planck's theory successfully explained the spectral distribution of energy emitted by a blackbody, which classical physics had failed to do.
Einstein's photoelectric equation:
Albert Einstein extended Planck's idea to explain the photoelectric effect, where electrons are emitted from a material when it's exposed to light.
He proposed that light incident on the metal surface as a stream of photon particles. The energy of each photon particle for light of frequency 𝜈, E = hv (where h = Planck's constant).
The incident photon collides with the electron of the metal. This collision can have two outcomes: either the photon is reflected with all the energy (h𝜈) or the entire energy (h𝜈) is transferred to the electron.
So it is clear that Einstein fully utilized the quantum theory of radiation to analyze the photoelectric effect.
When the entire energy (h𝜈) of the incident photon is transferred to the electrons of the metal, it is spent in two ways:
A fraction is spent ejecting the electron from the metal. Its minimum value is equal to the work function W₀ of the metal surface. However, more energy than W₀ is required to eject more electrons due to the interaction of positive and negative charges in the metal.
The remainder is converted into the kinetic energy of the emitted electrons. These moving electrons are photoelectrons, which can cause photoelectric currents.
If the energy taken by the electron to leave the metal surface is minimum i.e. W₀, then the emitted electron attains maximum kinetic energy (Emax).
If the mass of electron = m and the maximum velocity of photoelectron = vmax then,
So from equation no (1) we see
Again, if the value of the stopping voltage is V₀ for the light of frequency𝜈, we know that Emax = eV₀ (e = charge of the electron).
Hence, from equation (1), we see
Equations (1), (2) and (3) above are practically identical. So any of these equations is called Einstein's photoelectric equation.
Characteristics of the Photoelectric Effect
1. Photoelectric current is proportional to the intensity of incident light.
2. The maximum velocity or kinetic energy of the photoelectrons is not at all dependent on the intensity of the incident light; Rather, if the frequency of the incident light increases, their maximum velocity and kinetic energy increase.
3. Every metal has a threshold frequency. If light with a frequency lower than the threshold frequency falls on the metal surface, no photoelectric current is obtained. In general, the photoelectric effect is characterized by radiation from the wavelength of visible light to the wavelength of ultraviolet light.
4. The threshold frequency of the photoelectric effect is different for different metals.
5. Photoelectrons can be emitted from the metal surface at a range of velocities between zero and maximum velocities.
6. Photoelectric emission is an instantaneous process, i.e., photoelectrons are emitted as soon as light falls on the metal surface, there is no time lag between these two events.
7. Emission of photoelectrons in photoelectric emission causes the residual metal surface to become positively charged to a very small degree (this principle is adopted in making photo-voltaic cells).
8. Emission of electrons in photoelectric emission does not depend on the temperature of the metal.

Post a Comment (0)